The Lobular Breast Cancer Alliance (LBCA) was fortunate to be able to provide a travel grant to LBCA Patient Advocate Advisory Board member Julia Katherine Levine who served as a representative for LBCA at the San Antonio Breast Cancer Symposium (SABCS) in December 2022. Julia shared her experience as a three-time attendee of the symposium and as a patient advocate with metastatic lobular breast cancer representing LBCA at its partner Metastatic Breast Cancer Alliance’s strategic planning dinner. Below Julia summarizes the new invasive lobular carcinoma (ILC) research she thought most important to highlight.
1) Advocacy, Networking, and Socializing
The biggest (and most nerve-wracking) highlight for me was attending and presenting at the Metastatic Breast Cancer Alliance (MBCA) Strategic Planning Dinner as the metastatic representative from LBCA. I created and presented a slide in a lightning round talk on LBCA on what we have accomplished, our partnerships with metastatic organizations, and future projects on metastatic ILC. Member representatives from major metastatic organizations and pharmaceutical organizations presented as well. It was both moving and fun.
LBCA Board member Dr. Deb Mueller and her husband hosted a dinner at her beautiful house for LBCA advocates and SAB members. It was very nice and relaxing after a long day and gave me the opportunity to talk with some of the other LBCA grant recipients and meet our new SAB Chair Dr. Rinath Jeselsohn as well as lobular breast cancer researchers from Belgium, Drs. Christine Desmedt and Karen Van Baelen (the recipient of the 2021 ILC research grant LBCA awarded in conjunction with AACR).
The Alamo Breast Cancer Foundation program (and advocate lounge!) is an excellent way to meet other advocates and to learn about the science in lay terms. I attended as many programs as I could. The entire program is available online to Alamo registrants.
The NBCC Project Lead Advanced Topic from Dr. McAndrew, of UCLA, focused on the hottest issue of the year: “HER-2 low” positivity and results from the Destiny 04 clinical trial of the drug T-DXD (Enhertu), which is now approved for individuals with HER2 low Estrogen Receptor positive (ER+) metastatic breast cancer. It was explained that tumors considered HER2 low are those that score a 1 or 2 on Immunohistochemistry (IHC) testing and has found to be a status prevalent in up to 65% of individuals with breast cancer tumors that are ER+. He also discussed the follow up study looking at side effects. Interstitial Lung Disease (ILD) is the most serious side effect noted and the incidence of this side effect has been reported to be as high as 15%. Dr. McAndrew explained that the drug must be stopped right away if there are ILD symptoms. There was also discussion of the fact that there has been high discordance in the pathological determination of HER-2 low depending on who is performing the IHC testing. He noted that this suggests the need for testing refinements to ensure more concordance moving forward. The Trio trial will be studying T-DXD in patients with early-stage breast cancer. Dr. McAndrew noted that he had a poster at SABCS about that.
There were three Hot Topics sessions during which researchers and clinicians highlight their takeaways from the day, one of which included LBCA’s Founding SAB Chair Dr. Steffi Oesterreich. There were also interesting educational sessions presented by the Federal Drug Administration (FDA), the National Cancer Institute (NCI), and the American Association for Cancer Research (AACR). The FDA presenters discussed clinical trial exclusion criteria and why it takes a long time for them to update their requirements. The NCI presenters provided information on how to become an advocate with NCI. AACR’s session was about immunotherapy in breast cancer.
The cocktail party hosted by the organization Guiding Researchers and Advocates to Scientific Partnerships (GRASP) was also a great placeto see and network with other advocates and organizations. GRASP organized virtual poster walkthroughs of select posters that occurred via Zoom the week after SABCS. This year GRASP had four lobular-specific poster sessions focusing on two different ILC posters during each. LBCA advocates can sign up to become advocate participants or mentors for GRASP poster sessions that are being planned for SABCS23 and the next in person ILC Symposium in Pittsburgh, PA, next fall.
2) Posters
There were 22 lobular-specific posters this year and more that included or had relevance for ILC. There were six poster sessions throughout the week. Posters presented depict the findings of studies that have been submitted as abstracts for publication. They are not yet peer-reviewed and published manuscripts, though that is the next step for many. Because of other commitments it was impossible to see all of them in person, but when I did I always made an effort to introduce myself to the presenter, ask them to explain the poster, and in turn tell them about LBCA. I’m happy that there is a digital component to SABCS now as it gives one the ability to view the posters I had missed in previous years and to revisit ones later online that I visited during SABCS this time. The posters that focused on ILC research that resonated with me the most this year were the following:
Two Poster Surveys of patients and clinicians
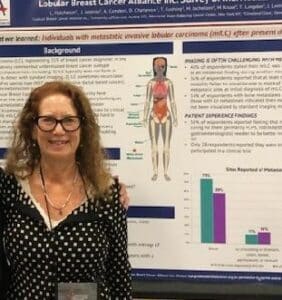
P6-05-50 (LBCA) Lobular Breast Cancer Alliance Inc. Survey of Individuals with Metastatic Invasive Lobular Carcinoma- highlights the need for better diagnosis, imaging and communication for metastatic lobular patients.
P6-05-10 (UPMC) An international survey on ILC reveals gaps in knowledge and top priority research areas. This poster was dedicated to LBCA’s late founder Leigh Pate.
A poster on Rapid Autopsy/Tissue Donation programs
P6-14-14 (KU Leuven) Advancing research on metastatic breast cancer: the UPTIDER post-mortem tissue donation program. A tissue donation program in Leuven, Belgium. Initial results highlighted the vast underestimation of disease extent on imaging during life.
Other posters I found interesting pertained to ER Resistance and Circulating Tumor Cells (CTC’s). (Note: lay summaries of three of the following can be found on the LBCA website):
P4-01-24 Targeting receptor tyrosine kinases in overcoming tamoxifen resistance and dormancy in invasive lobular cancer
P3-05-08 Prevalence and prognosis of ER-loss in advanced invasive lobular carcinoma
P4-02-04 Serial monitoring of circulating tumor cells and circulating tumor DNA in metastatic lobular breast cancer identifies intra-tumor heterogeneity and precision and immuno-oncology biomarkers of therapeutic importance
P1-05-23 Optimizing the diagnosis of leptomeningeal metastases in breast cancer patients by circulating tumor cells and circulating tumor DNA- (highlighting the utility of using less invasive CTC’s instead of CtDna to diagnose Leptomeningeal disease earlier)
3) Scientific Sessions
Challenging Types of Breast Cancer – (the single scientific session with a specific lobular segment)
LBCA SAB researcher Dr. Jorge Reis-Filho from Memorial Sloan Kettering Cancer Center spoke on histopathological special types of breast cancers including the pathology and genomic aspects of different subtypes in ILC. Dr. Sibylle Loibl, German Breast Cancer Group, presented an overview on lobular and pleomorphic breast cancer. Included were slides on the most recent mutational landscape of ILC, genomic targets in Pleomorphic ILC, a recent meta-analysis of surgical procedures, and a review of the studies on both adjuvant and neo-adjuvant chemotherapy and hormonal treatment in ILC. A review of current clinical trials revealed the disappointing news that the GELATO trial in the Netherlands, one of only two metastatic ILC clinical trials anywhere, has been discontinued due to lack of benefit. (Note: SABCS22 registrants can view all slides from the SABCS22 scientific sessions online by logging into the SABCS22 virtual platform.)
This year the amount of lobular content in the programming was underwhelming and somewhat disappointing, unlike two years ago when there was an entire Spotlight Session devoted to ILC. Many noted this was a problem and one of the co-presidents of SABCS acknowledged this on Twitter and said that they would try to do better. The surprising silver lining was the Year in Review session where Dr. Marlene Kok, Netherlands Cancer Institute and lobular researcher, noted the lack of lobular research and trials and made the suggestion that there always be a discussion of lobular breast cancer in the end of year round-up.
Clinical Trial Updates and New Drugs on the Horizon for metastatic ER+ BC (not ILC-specific) I found Notable
- CAPITELLO-291– It is a Phase 3 trial of Capiversitib, an AKT inhibitor that will target the PIK3CA pathway. It claims to have less toxicity and side effects than Alpeisib (Piqray ), which is the drug currently approved for PI3KCa mutations.
- EMERALD – It is a Phase 3 trial of Elacestrant – an oral Selective Estrogen Receptor Degrader (SERD). It is claimed that the drug has better bio-availability (it can be absorbed better by the body) than, and may be able to be used in place of, Fulvestrant (injection) alone or in combination with other drugs. Results reported so far have shown efficacy in patients with ESR1 mutations. Approval of this drug is anticipated in 2023. Other similar trials and drugs reported on that seemed promising include a drug in the Phase 2 SERENA 2 trial: Camizestrant (AZD-9833) and a drug in a Phase 2 VERITAC Trial: ARV-471 (PROTAC). Approvals of these drugs is further off.
- RIGHT CHOICE – Phase 2 trial – Information presented claimed that “For patients with HR-positive, HER2-negative advanced breast cancer and high tumor burden or aggressive disease features who were treated with ribociclib plus endocrine therapy (ET) had fewer adverse events and significantly longer progression-free survival than those treated with combination chemotherapy In first line setting.”
- STIC-CTC – Phase 3 trial – Results presented claimed that the use of circulating tumor cell (CTC) count as a guide to first-line treatment, either with chemotherapy or endocrine therapy, led to better overall survival compared with physician’s choice of treatment without CTC.
- Discussion of CDK4/6 Resistance: Clinical – session title: View from the Trenches – What to do on Monday Morning [referring to right after SABCS22 has concluded]. My impression from this session was that it appears that currently there is limited and conflicting data to guide treatment decisions after progression on first line treatment (i.e., the first treatment prescribed that is thought to be the best for the circumstance) with a CDK4/6 inhibitor drug. Dr. Fehm, Henriech-Heine University, presented data on various trials and options as follows:
-
- MAINTAIN trial showed a progression-free survival benefit for ribociclib and switching ET after failing existing CDK4/6 inhibition.
- PADA1 trial showed improved PFS by changing treatment from AI/palbociclib to fulvestrant/palbociclib at the onset of ESR1 mutation with dynamic ctDNA monitoring.
- PACE trial showed improved PFS by changing the current ET to fulvestrant and adding Avelumab, an anti-PD-L1 immune checkpoint inhibitor. Palbociclib + fulvestrant alone did not boost PFS. “The most promising results come from switching the ET backbone and switching the CDK4/6i to ribociclib, with anecdotal evidence for abemaciclib.”
- Year in Review Discussion of CDK/4/6 Resistance: Basic Science Dr Goga of UCSF reported on the basic science of CDK4/6 resistance and ways we might overcome them. Dr. Goga noted that “two strategies to overcome CDK4/6i resistance have emerged.” He described that the focus of these studies begins with the recognition that there are multiple cyclin dependent kinases.
4) TRANSLATIONAL CONTROVERSIES
Session title: RIP MTD: Dr. Rugo discussed the need for research trials to move away from the goal of identifying a Maximum Tolerated Dose (MTD) and instead move towards identifying the most manageable optimal dosing or a Minimum Effective Dose. MTD can lead to intolerable dosing and discontinuation. This topic is very important to metastatic patients and is in large part a result of The Right Dose, a patient centered advocate initiative (PCDI). Clinical trials are also now starting to use different doses to determine the optimal tolerated dose.
Session title: Randomized clinical trials vs. real world evidence: Dr. DiMichele of UCSF discussed using real world evidence (RWE) as well as randomized trials in clinical practice: “Trial populations lack real-world diversity. Incorrect assumptions based on trials that lack diversity can derail accurate interpretation of findings and leave clinically important questions unanswered. RCTs take too long to get new drugs to the patients who need them and cost too much. We should require RWE as part of the drug approval process.”
5) Patient Perspective: Living with Metastatic Breast Cancer
Stephanie Walker, MBCA and the BECOME initiative founder, spoke on health disparities and racial inequalities, clinical trials for underserved communities, quality of life and survivorship, and the importance of education and support in metastatic breast cancer. Christine Hodgden, GRASP co-founder, spoke about her patient and public policy perspective on breast cancer research. She discussed advocacy, funding metastatic breast cancer research, research advocacy, and rare and special breast cancer populations including lobular breast cancer. Both spoke on the importance of including the patient and advocate voice in metastatic breast cancer research. Both presentations highlighted the many organizations working towards these goals including LBCA, the European Lobular Breast Cancer Consortium (ELBCC/Lobsterpot), and Lobular UK.

