FAQs
Frequently Asked Questions About ILC
These Frequently Asked Questions (FAQs) and their answers about invasive lobular carcinoma (ILC), also known as lobular breast cancer, are for informational and educational purposes only to assist patients and caregivers with learning about ILC. It should be noted that the answers provided in this document reflect the prevailing opinion in the U.S. The information is not meant to replace the advice and information patients receive from their health care providers. More research and clinical trials are needed to improve outcomes for systemic treatments such as endocrine treatment, chemotherapy, immunotherapies, imaging, and targeted treatments, and for potential future targeted therapies or immunotherapy that might result in better long-term outcomes for ILC patients. Scroll down to read all the FAQs. You can also download an FAQs document.
We encourage you to learn more about LBCA’s ILC research priorities.
ILC is the second most common histologic subtype of breast cancer after invasive ductal cancer (IDC), which is also known as invasive ductal carcinoma of no special type (IDC/NST). ILC is often a low grade and indolent proliferative cancer, meaning that the cancer cells look more like normal cells and the cancer tends to grow and spread slowly. Like most breast cancers, ILC tends to be estrogen receptor (ER) positive, progesterone receptor (PR) positive, and human epidermal growth factor receptor 2 (HER2) negative. ILC can sometimes be triple negative (lacking expression of ER, PR and Her2) or HER2+, but this is rare. It is evident that the clinical behavior and molecular features of ILC are distinct from IDC.1, 2, 3 One of the important features of ILC is the loss of the ability of the cancer cells to “stick together” referred to as “cell-to-cell adhesion.” The lack of cell adhesion means that ILC does not form a mass the way other breast cancer tumor types like IDC/NST do. Rather, the tumors may grow in what is referred to as a “diffusely infiltrating” manner or more plainly, as lines of separate cells. In ILC, the inability of cells to stick together is due to the loss of a functioning protein called E-cadherin. 4, 5 Loss of E-cadherin is often due to an inactivating mutation in the CDH1 gene. The way ILC tumors grow can make them harder to feel on examination, to image, and to diagnose.1, 3, 6 Tumors can therefore be larger and/or more advanced at the time of diagnosis. In addition, there are other molecular characteristics that are either more prevalent or less frequent in ILC versus IDC. 7, 8
ILC is the second most common histological type of breast cancer diagnosed, accounting for about 10-15% of all breast cancers. 9 An estimated 43,000 new cases of ILC are diagnosed each year. 10 ILC impacts more women than do cancers of the kidney, brain, pancreas, liver, or ovaries. 11 The incidence of all types of breast cancer has been increasing 0.5% annually since 2004.12
Classic ILC is the most common subtype of lobular breast cancer, but there are other subtypes of ILC such as Pleomorphic ILC (less than 5% of ILC),13 which often has an increased proliferation index (Ki67) and higher grade, meaning that the cancer grows more quickly and aggressively than Classic ILC. Pleomorphic ILC also exhibits less hormone receptor positivity, and greater overexpression (positivity) of HER2 than classic ILC. Other less common ILC subtypes include tubulo-lobular, solid, and alveolar, which have different cellular and microscopic characteristics.2
IDC/NST is the most common type of breast cancer. IDC/NST cancer cells have a different appearance, tumor activity and biology/behavior than ILC. Unlike ILC, most IDC/NST tumors express E-cadherin and tend to form a lump in the breast. Some patients with metastatic ILC can have a higher likelihood of metastases going to organ sites that are less common than the sites that IDC/NST will metastasize to, such as the gastrointestinal tract.14 ILC patients tend to be older, have larger tumors at diagnosis, present at a later stage and have higher numbers of positive nodes. These unique features of ILC present challenges in initial diagnosis, imaging for staging, and clinical trial enrollment.
Although primary ILC tumors can be lower grade and proliferation compared to IDC/NST tumors, they can still recur after initial treatment. Recent studies suggest higher propensity for late recurrence after 5 years in patients with ILC compared to those with IDC/NST. There are some studies that show that some patients with ILC may have a slightly worse survival rate compared to patients with IDC/NST, but there are not yet studies that indicate specifically which subpopulations of patients with ILC this applies to.15, 16
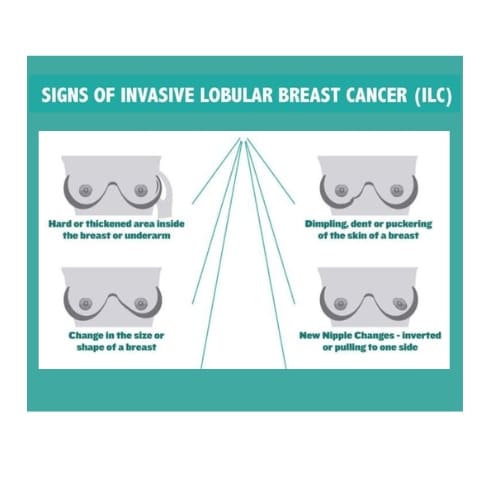
Symptoms of primary ILC in the breast can range from none to visible changes in the breast tissue. On self-examination, ILC can sometimes be felt as a firmness or mass. Routine self-exams are encouraged so that changes or differences can be felt/noticed. Any new firmness or masses should be further evaluated. Breast Cancer, including ILC, may at times cause a visual puckering or pulling of the skin, flattening or inversion of the nipple, unexplained skin hardening, or a visual dimpling or dent in the breast. One breast may appear larger or have a different shape than the other. Occasionally, a patient may present with a shrunken breast as the tumor pulls on the tissue around it. Breast pain is a less common occurrence. ILC may not be detectable on self-exams at all, even when the tumor is large. 3, 17 Enlarged lymph nodes may or may not be felt in the axilla (underarm area). If you have any suspicious findings, report them to your doctor immediately.
While mutations in certain genes can increase the risk of developing breast cancer, specific hereditary mutations in the CDH1 gene can confer a lifetime risk of both ILC and hereditary diffuse gastric cancer (HDGC). Most patients with ILC do not have this mutation and this mutation is rare. 18 More often, patients may be referred for genetic testing at diagnosis if there is a personal or family history of breast cancer, 19, 20 if patients are <50 years of age at diagnosis, and depending on personal family history and ethnicity.21 Patients with lobular breast cancer in both breasts under 50 years of age, or one breast who have a family history of lobular breast cancer and are diagnosed before the age of 45, may be tested for CDH1 mutation specifically.22 Mutations in other genes including BRCA1, BRCA2, CHEK2, and PALB2 also increase the risk for breast cancer.23
Atypical lobular hyperplasia and LCIS are not considered cancer, however both lesions are composed of abnormal cells that share features of the cancer cells that are seen in ILC. Hyperplasia is an overgrowth of cells. “Atypical” hyperplasia means that the cells look abnormal. In both of these lesions, the abnormal cells are growing within the lobules (milk glands) or ducts of the breast but have not yet started growing through/outside the wall of the lobules/ducts. The diagnosis of these lesions in the breast is associated with an increased risk of developing breast cancer in either breast. We also consider these lesions as ‘non-obligate precursors to invasive breast cancer,’ which means patients with ALH or LCIS may never develop cancer. These pathology findings are therefore viewed as markers of increased breast cancer risk.24
LCIS indicates that the patient is at increased risk and can develop breast cancer in either breast (not necessarily ILC). The World Health Organization (WHO) now classifies 3 variants of LCIS: classic (CLCIS), florid (FLCIS), and pleomorphic (PLCIS). These non-invasive variants share some molecular characteristics with the invasive variants of the same name, suggesting a similar cellular origin. At many institutions, patients with Classic LCIS are not referred for excision (i.e., surgery) due to low upstage rates (i.e., low instances of becoming invasive). The pleomorphic and florid variants of LCIS are thought to be genetically and biologically more advanced lesions than classic LCIS. CLCIS, florid LCIS, and pleomorphic LCIS diagnosed on core biopsy are sometimes treated with surgical excision. Following surgery, the final diagnosis may be upgraded to invasive cancer in as many as 40% of these samples.25
Histology refers to what is seen when cells are examined using a microscope. Histological subtypes are the smaller groups that a cancer, such as ILC, can be divided into based on certain characteristics observed under a microscope.
Receptors are proteins that are found on the surface of a cell or within a cell. These receptors bind to very specific proteins (ligands). Examples of receptors that are important in breast cancer are estrogen and progesterone receptors (ER or PR), or the human epidermal growth factor receptor 2 (also called HER2). When ligands bind to receptors, specific cellular activities occur, for example stimulation of cell growth or migration. When cells are receptor “positive,” it means that the cancer cells express lots of this receptor. This is important in breast cancer, because a cancer that is HER2+ or ER+ (“positive”) means cancer cells will most likely respond (be killed) to targeted therapy against HER2 or ER, respectively. “Negative” means they will not. If a cancer is hormone receptor (ER and PR) negative and HER2 negative, it is considered to be triple-negative breast cancer (TNBC). Androgen receptor (AR)26, 27positivity and HER2 low positivity28 have become areas of study for targeted treatment in breast cancers.
An IHC (ImmunoHistoChemistry) test is a special staining process performed on breast cancer tissue removed during a biopsy or from surgery. IHC testing is performed by pathology departments during breast cancer diagnosis, and it is used to see if the cancer cells express estrogen (ER), and progesterone (PR), and human epidermal growth factor receptor 2 (HER2). It also helps to specify whether a breast cancer is ductal or lobular, using the expression or absence of the protein E-cadherin. Finally, it can aid in determining how fast a tumor is growing, using markers such as the protein Ki67 or MIB1.
Ki-67 is a protein that increases as cells prepare to grow (divide). A staining process can measure the percentage of tumor cells that are positive for Ki-67. The more positive cells there are, the more quickly they are dividing and forming new cells, and so it can be used as a sign of the cancer growth rate. Ki-67 levels are not tested routinely but, in some cases, may help guide your team’s treatment decisions.
Breast density reflects the amount of fibro-glandular tissue compared to the amount of fat tissue in the breast. Women with dense breast tissue have a 2-fold risk of developing any type of breast cancer. There are no data regarding whether density increases the risk of specific breast cancer types, such as lobular. A mammogram report categorizes the density of tissue from BI-RADS 1 to 4, four being “extremely dense.” Women with moderate (category 3) to extremely dense breasts (category 4) may be offered supplemental screening methods, as ILC is difficult to detect in dense breasts.29
The use of post-menopausal hormone replacement therapy with both progesterone and estrogen has been associated with an increased risk of breast carcinoma. While there have not been large studies to determine whether use of HRT increases risk of lobular breast carcinoma, one study that compared nonusers of HRT with those using combined estrogen and progestin hormone replacement therapy (CHRT), found that those using HRT for at least 6 months had an elevated risk of lobular breast carcinoma.30
Screening mammograms are performed on women who have no signs or symptoms of breast cancer, and who have not had a breast cancer diagnosis in the last three years. General population breast cancer screening is intended to detect unsuspected early-stage breast cancers. Diagnostic mammograms are typically performed on women who have a recent history of breast cancer, symptoms or abnormal physical exam findings, or who are considered to be at higher risk for breast cancer for follow-up when abnormalities are identified on screening mammograms).
ILC often grows in a linear pattern through the breast without changing the surrounding structures or forming a discrete mass or lump. This is the reason why ILC can be more difficult to detect than IDC/NST on mammography and ultrasound. Dense breast tissue further decreases the sensitivity of mammograms, to as low as 11% in some studies of ILC.3, 31
Ultrasound (US) can be used for supplemental imaging in women with dense breasts or those at increased risk for breast cancer. In women with normal mammograms, US detects an additional 3.5 cancers per 1000 women screened.
Magnetic resonance imaging (MRI) is recommended for women with a greater than 20% lifetime risk of breast cancer, such as those with BRCA1 and BRCA2 genetic mutations, strong family history of breast cancer, personal history of breast cancer at a young age, and in breast cancer survivors with dense breasts. It has the highest sensitivity of all imaging modalities for detecting breast cancer.32 MRI can also be useful for preoperative staging of some breast cancers. In a recent study using MRI prior to surgery for lobular breast cancer, 21.5% of lobular patients changed surgical plans from breast conserving surgery to another operation as a result of pre-operative MRI findings. The final surgical pathological correlation was better with MRI than with ultrasound or mammogram. MRI can identify multifocal areas of the tumor not seen on mammogram, and this information can be helpful in choosing the extent of surgical intervention.33, 34
Contrast enhanced spectral mammography (CESM) is a special mammogram that uses contrast to assist with localizing the size and extent of breast tumors. Several small studies have shown that it is superior to 2D mammograms as well as MRI in sensitivity and false positive results and may be especially useful for screening in women with a history of breast cancer and dense breasts, or women at intermediate risk for breast cancer.35, 36 Studies to replicate and validate these findings are underway. As a result, CESM is not widely available and not part of standard of care.
Molecular breast imaging (MBI) is another technique that uses radioactive tracers to highlight abnormal tissue that is taken up by the tracer. It is nearly comparable in rates of detection to MRI, and especially useful in patients with dense breast tissue.37 However, the images of each breast take ten minutes, and the radiotracer exposes the entire body to radiation, unlike other breast imaging modalities. MBI can be particularly helpful in women with dense breasts and may be helpful when MRI is not available/possible, but at this time there is no clinical consensus on indications for use and this imaging is not part of standard of care.38 Few centers utilize MBI when MRI is available.
After completion of treatment, it is typically recommended that yearly mammography should be performed as it has been demonstrated that in this population it significantly improves breast cancer survival.39 In cases in which mammogram missed the ILC, supplemental imaging such as US and MRI may be recommended. As everyone’s circumstance is different the ultimate decision will be decided between the patient and her care team.
An individual’s treatment plan for ILC depends on many factors, including the size and grade of the cancer, genetic factors, lymph node involvement, and the patient’s overall health and individual preferences. At present, there are no ILC-specific treatment guidelines. The standard of care for early-stage treatment of hormone receptor positive ILC is to the same as treatment of hormone receptor positive IDC/NST. Recommended treatment will likely include surgery (lumpectomy or mastectomy), radiation, and systemic therapies such as chemotherapy or hormonal therapy.
Surgery
Surgical planning takes into account how best to remove all of the cancer (i.e., achieving “clear margins” around the tissue removed). If the size of the tumor is too large to remove without leaving sufficient cancer free margins, or sufficient normal breast tissue, then a mastectomy may be recommended.3 Oncoplastic lumpectomy, a special technique that removes more tissue, has also been studied as an option to clear the margins more effectively in ILC.40 Initial breast conserving therapy may require additional surgery if margins are positive, to remove further cancer tissue. This is particularly true for lobular cancer, which more often presents with diffuse disease and multifocal lesions (several areas of cancer involvement within the breast) that may be difficult to detect with pre-operative imaging and during surgery.41 Long-term data suggests that choice of surgery (lumpectomy vs. mastectomy) does not affect long term survival.42, 43
Neoadjuvant therapy (systemic medical treatment prior to surgery) followed by lumpectomy can be an alternative option to decrease tumor size to facilitate surgical removal, and to evaluate the tumor’s response to therapy prior to removal to help guide treatment after surgery.
Post-mastectomy surgical options include aesthetic flat closure (i.e., no reconstruction), breast implants, or flap surgeries that use the patient’s own fat or muscle to create new breasts. Patients are usually referred to plastic surgery to discuss post-mastectomy options.
Radiation
Radiation therapy, also known as radiotherapy, uses high energy beams to kill the genetic material within cancer cells. An individual’s treatment plan may include radiation based on whether they have a lumpectomy or mastectomy, the location of the tumor, and other factors.
External beam radiation therapy (EBRT) focuses radiation to a specific area of the body such as the breast, supraclavicular area (chest wall) and the axilla (underarm area) after positive lymph nodes are removed or identified. This can destroy any remaining microscopic areas of cancer, to prevent local recurrence.
Partial breast radiation (PBI)44 and intraoperative radiation45 have been studied as alternatives to ERBT but has not been conclusively proven to be as effective for routine use in ILC.
Medical Therapies
The main reason that medical treatment, also known as systemic therapy, is prescribed after tumor removal is to prevent recurrence of cancer, both in the breast and in other areas of the body. There are many different categories of medical treatments including endocrine therapy, chemotherapy, immunotherapy, and other targeted therapy.
Endocrine (anti-hormonal) therapies
As many lobular cancers are hormone receptor (ER) positive, endocrine therapies are a mainstay of treatment for this type of cancer. Aromatase inhibitors (AIs) are often prescribed to post-menopausal women, and tamoxifen to pre-menopausal women. Some premenopausal women will receive tamoxifen or an AI, in conjunction with ovarian suppression, particularly those patients with high risk of cancer recurrence. A large retrospective trial comparing letrozole to tamoxifen showed letrozole is superior for patients diagnosed with lobular versus ductal carcinoma, but this needs further validation. 46 A current clinical trial is studying whether a specific endocrine therapy might be superior in post-menopausal women with ILC.
Standard duration of endocrine therapy is five years although determining the optimal duration of endocrine therapy is an area of active research; the Breast Cancer Index along with other factors (tumor size, grade, and lymph node involvement) can help guide decisions to extend endocrine therapy beyond five years. 47, 48
Chemotherapy
Chemotherapy decisions are determined by multiple factors, including clinical and pathological features such as tumor size, positive or negative lymph, tumor grade, tumor markers and molecular prognostic testing such as Mammaprint or Oncotype DX. Prognostic testing may help determine whether a particular cancer will benefit from chemotherapy, however, the utility of these tests for ILC specifically has only recently emerged.49 One study reported lower pathologic complete response in ILC than IDC when chemotherapy is given to patients prior to surgical treatment (neoadjuvant chemotherapy).50 Retrospective studies have shown some differences in response to systemic therapies between ILC and IDC.51 Similar to patients with IDC, patients with ILC with specific high-risk factors such as triple-negative subtype or high-grade tumors tend to have a better response to chemotherapy.52
Targeted Therapies
Endocrine therapy is the most commonly used targeted therapy. Additional targeted therapies may be used along with endocrine therapy and others are used when endocrine therapy is not appropriate. Some targeted therapies are used only for Stage IV or metastatic breast cancer while others can be used for earlier stage cancers.
- Cyclin-dependent kinase 4/6 (CDK4/6) inhibitors are oral targeted therapies that block a protein called cyclin-dependent kinase, causing the tumor cells to stop dividing and making new copies of themselves. These inhibitors may be used in conjunction with endocrine therapy to treat advanced or metastatic, ER positive/HER2 negative breast cancer. There is only one CDK4/6 inhibitor approved for use in some specific cases of early breast cancer. Certain patients with a defined high-risk profile may be advised to receive this therapy.53
- Immunotherapies (including checkpoint inhibitors and other types of immune therapies) are medications that affect cancer cells’ ability to avoid the immune system. One commonly used drug has been approved for early stage as well as metastatic triple negative breast cancer. Active research is being done to determine if there is a role for use of immunotherapy in ER positive and HER2 positive breast cancer.
- Poly (ADP-ribose) polymerase (PARP) inhibitors are oral medications used specifically in patients with HER2 negative breast cancer with BRCA1 or BRCA2 mutations.54 PARP proteins are involved with DNA repair, and function abnormally with BRCA mutations. There are currently two PARP inhibitors commonly used for metastatic breast cancer treatment. One of them is also approved as part of treatment for high-risk early-stage breast cancer in those patients with BRCA mutations.55
- Monoclonal antibodies, and Antibody drug conjugates (ADCs) are used in HER2 positive breast cancer to target the HER2 protein. One of the antibody drugs is now also now approved for treatment of HER2-low metastatic breast cancer. 56
- PIK3CA inhibitors block a key signaling pathway in breast cancer. Currently the only approved PIK3CA inhibitor is alpelisib indicated for patients with metastatic ER positive breast cancer who have PIK3CA mutations.
For more information about targeted therapies visit https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies
ILC is most often an estrogen sensitive disease. Patients with ER positive ILC should consult with their care team before taking prescription or homeopathic medications or supplements that contain or mimic the hormone estrogen, including hormonal replacement therapies to relieve menopausal symptoms, supplements or other products that contain or mimic estrogen.57 General healthy lifestyle recommendations for breast cancer recurrence prevention also apply to patients with ILC, including a healthy diet, avoidance of weight gain (estrogen is produced in fat/adipose tissue), exercise, no alcohol consumption, and stress reduction. Exercise, in particular, has been shown to have a positive effect on long-term survival and breast cancer specific mortality.58
No. Complementary and alternative medical treatments (CAM) for breast cancer include mind-body therapies such as meditation and biofeedback; biologically based practices such as vitamins, herbs, and dietary changes; manipulative practices such as massage and chiropractic treatment; and other healing systems, including Ayurvedic, Chinese, Naturopathic and Homeopathic Medicine. While some CAM therapies are generally safe and may ease the discomfort of cancer treatment, other CAM therapies can be harmful, particularly if standard medical treatments are not used.59 Several studies have shown that women who rely solely on CAM for their breast cancer treatment have worse outcomes.60, 61, 62, 63, 64
V. Metastatic Lobular Breast Cancer FAQs
Breast cancer that has spread beyond the breast and local lymph nodes is considered stage IV, or metastatic breast cancer. Metastatic breast cancer is treatable but not considered curable. With targeted therapies and chemotherapy, patients with metastatic lobular breast cancer can often live for many years. Some patients are initially diagnosed with cancer already at stage IV (de novo metastatic ILC), while for others, it can recur years later at distant sites/other organs (distant recurrence metastatic ILC). If the original breast tumor pathology was ILC, tumors found at distant sites away from the breast are usually also ILC, however, metastases can sometimes be heterogeneous (different tumors may have different characteristics) or can recur as ILC with a different receptor status such as HER2 + or triple negative features. 65
Like ductal breast cancer or breast cancer NST, lobular breast cancer can recur any time after initial diagnosis. It can also be first detected/diagnosed as stage IV (referred to as de novo disease) and appear first in sites other than the breast (e.g., bones, liver, lungs), even when cancer has not yet been detected in the breast. Studies show that ILC often recurs later than IDC, significantly more so after ten years after the initial diagnosis of cancer.16
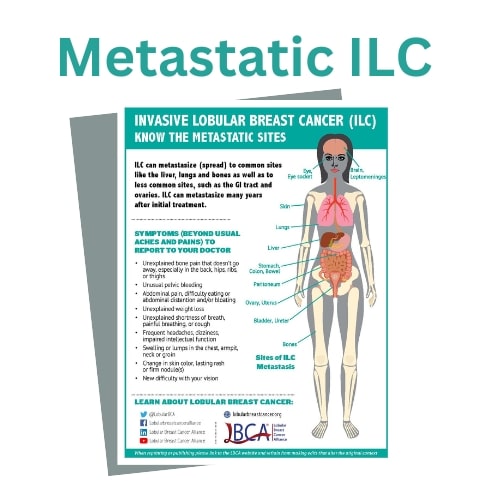
Similar to IDC/NST, ILC can metastasize to the lymph nodes, bones, lungs, liver, and the brain. However, ILC can also spread to unique sites such as the gastrointestinal tract (stomach, small intestine, and colon), gynecological organs (ovaries, uterus), the peritoneum (abdominal lining), and in rarer cases leptomeninges (lining of the brain and spinal cord), 66 and orbital tissues (tissues around the eye).67 The most common site of ILC metastases is bone. Lung and liver metastases are less common in ILC than in IDC/NST. 68, 69 Other unusual locations are listed on the LBCA website.
Some potential symptoms of metastatic breast cancer to report may be bone pain, abdominal pain, distention and/or bloating, shortness of breath, pelvic bleeding, headaches, or changes in vision or the appearance of the eye. Because ILC can metastasize to uncommon sites, it is important for patients with lobular breast cancer and their oncologists to be aware of these differences, and to discuss the importance of recognizing and reporting other possible symptoms referring to these unusual sites of metastasis.
The type of imaging studies and scans used to identify metastatic ILC depends on the location of the metastatic sites. The available imaging options are currently the same as those for imaging ductal breast cancers. They include, CT scans, bone scans, FDG PET scans, FES PET scans, and MRI. Bone scans look for evidence of bone remodeling, which often occurs at sites of bone metastases. FDG-PET uses glucose metabolism uptake in the body and is a sensitive method for detecting, staging, and monitoring the effects of therapy. However, it may be less sensitive at detecting ILC lesions than IDC/NST lesions.70 CT scans, PET scans and/or MRI may be used for identifying lesions in the liver, and MRI can be used to visualize other areas such as the brain for metastasis.71 A newer radio-pharmaceutical agent, F18 Fluroestradiol (FES), targets the estrogen receptor, and may be used in some cases to image ER+ breast cancer. This imaging agent has been FDA approved specifically for imaging recurrent or metastatic breast cancer. FES scans are proving to be helpful in visualizing lobular tumors in certain parts of the body, and other use of FES scans is being studied in clinical trials.72 In order to ensure accurate FES testing, patients may not be on Fulvestrant or tamoxifen at the time of the test. FES is less effective in detecting lesions in the liver.73 Whole body MRI is under investigation in research studies as it may be valuable to help visualize ILC when traditional CT and FDG PET scans are not useful.74
Currently, metastatic ILC is treated in the same manner as other types of metastatic breast cancer based upon its subtype. Patients with hormone receptor positive metastatic breast cancers receive the same treatment whether they have metastatic IDC/NST or ILC, where anti-Estrogen drugs, Targeted Therapies and Chemotherapy are standard of care treatments. In limited studies, CDK4/6 inhibitors and one type of chemotherapy were shown to work as well in ILC as in IDC/NST.75, 76 Similarly, patients with HER2+ or Triple Negative metastatic breast cancer are treated according to treatment guidelines whether the patient has ILC or IDC/NST, using HER2-targeted therapy and/or chemotherapy. In 2020, The American Society of Clinical oncologists (ASCO) published the types of treatment available for all subtypes of metastatic breast cancer.77 This online resource continues to be updated as research changes with regard to treatment options.
Surgery to the primary tumor in de novo metastatic disease is controversial.78 A large randomized trial done in the United States determined that there was no overall survival benefit to removing the primary tumor but is helpful for local control of cancer complications including skin breakdown/infections/pain or preventing recurrence in the breast.79 Several other international retrospective studies have shown that there may be an overall improved survival benefit in select patients such as bone only oligo-metastatic disease (5 or fewer metastases in one organ).80, 81 Primary surgery in de novo metastatic disease is a personal medical decision depending on the individual and their circumstances and the decision should be made in discussion with the clinical team.
Biopsies are often recommended to confirm the diagnosis of distant metastatic disease when feasible (if the metastatic site is accessible). A biopsy can determine whether the cancer has changed into another subtype (i.e. an ER+ primary cancer can become an ER- metastasis) which can happen in approximately 20% of cases due to treatment resistance) and help to determine future treatment options.65 Colonoscopy and/or endoscopy may identify abdominal or colon metastasis if symptoms are not explained by imaging findings, and cerebral spinal fluid sampling (CSF) or “spinal tap” can help to confirm a diagnosis of metastatic ILC or of leptomeningeal disease, specifically.66
Biomarker testing in tissue from a biopsy or by liquid biopsy (blood test) may be performed to identify genomic mutations in metastatic tumors either at initial diagnosis, or upon disease progression. Most ILC tumors harbor a genomic CDH1 mutation for which there is currently no targeted treatment. Approximately 40% of lobular tumors harbor a PI3 Kinase (PIK3CA) mutation, for which there is an approved targeted treatment: Alpelisib for ER+ metastatic patients. Approximately 5% of lobular tumors harbor mutations in the HER2 gene (ERBB2), affecting the HER2 pathway.82 Neratinib (an approved drug for HER2+ breast cancers) is being studied for treatment in metastatic ER+ breast cancers, including patients with ILC, with ERBB2 mutations in ongoing clinical trials.83, 84 Biomarker testing may also be useful in determining anti-estrogen resistance such as the finding of ESR1 (the gene coding ER) mutations85 and other mutations such as RB1 which may confer resistance to CDK4/6 inhibitors.86 In other studies, ILC metastasis is showing a higher TMB (tumor mutational burden) score relative to IDC/NST. It is thought that this could prompt the development of further treatment options using immune checkpoint Inhibitors. Patients with metastatic disease and a high tumor mutational burden are currently candidates for treatment with the immune checkpoint inhibitor, pembrolizumab.
Tumor markers in the blood such as CA15-3 or CA27.29 are sometimes used for tracking metastatic disease progression or response to therapy.
There are many clinical trials available for patients with metastatic breast cancer and many for ER+ metastatic breast cancer. Some of these trials may have lobular cohorts or subsets of ILC patients in their final analysis. There are only a few trials that specifically target metastatic ILC.87 There are some ongoing imaging trials including metastatic lobular breast cancer patients, and European trials specifically studying treatment effects in ILC. See LBCA website for current ILC Clinical Trials. One of the primary challenges in evaluating efficacy in clinical trials with lobular metastatic patients is the RECIST criteria (response evaluation criteria in solid tumors) required by most trials.88 Due to the diffuse pattern of ILC, it may not form a measurable mass to follow, making these patients ineligible for many trials. In a recent review, patients with metastatic invasive lobular carcinoma were shown to be significantly underrepresented in breast cancer clinical trials.89 A second key challenge in conducting clinical trials that include a metastatic ILC cohort is the lower percentage of patients with ILC as compared to IDC, therefore it is important for collaboration across institutions and multicenter trials that include lobular cohorts to increase the numbers of lobular patients in trials. Clinical trial enrollment can help advance research and has been shown to lead to longer overall survival.89
VI. Additional FAQs/Resources
There are many online tools and links to find research studies and clinical trials such as Clinical Trials.gov, breastcancertrials.org * or metastatic trials tool*. Most clinical trials are not ILC specific, but could include or have a cohort of patients with lobular breast cancer. There are a few ILC specific trials listed on the LBCA website.
Although some oncologists research and study ILC, they do not consider themselves specialists and there is not currently a lobular clinical specialty. LBCA also does not recommend specific health providers or treatment facilities regardless of specialization. LBCA suggests that if not already receiving care at one of the National Cancer Institute (NCI) Designated Cancer Centers around the country, individuals might pursue this or consider second opinions at such a center if accessible. These Cancer Centers treat larger volumes of patients, see more lobular breast cancer patients, and serve as centers for research and cutting-edge cancer treatments.
Second opinions are always an option and may provide some reassurance or clarity around diagnosis and treatment information. Whether and when to seek a second opinion is a personal decision. Second opinions can be sought for treatment advice, for radiology interpretation, and for pathology review. With recent advances in telemedicine, virtual second opinions may be possible. The National Cancer Institute provides some useful guidance on when you may consider seeking a second opinion.
For more information and resources visit LBCA’s website, lobularbreastcancer.org. Through our website and social media we provide a platform for presenting and discussing current ILC research and findings, webinars, videos and blogs on ILC topics and advocacy training, and an online community for individuals who have been diagnosed or are living with ILC. We also maintain a library of current lobular breast cancer studies, identify clinical trials enrolling individuals with ILC, and provide ILC patient advocacy opportunities and tools with which patients and caregivers can learn to become advocates to advance research, raise awareness, and educate others about ILC. LBCA provides an advocacy toolkit for this purpose. LBCA also hosts a Facebook page and publishes a monthly newsletter to which you can subscribe.
1 Ciriello G, Gatza ML, Beck AH,et al Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell. 2015 Oct 8;163(2):506-19. PMID: 26451490
2 McCart Reed AE, Kalinowski L, Simpson PT, Lakhani SR. Invasive lobular carcinoma of the breast: the increasing importance of this special subtype. Breast Cancer Res. 2021 Jan 7;23(1):6. PMID: 33413533
3 Wilson N, Ironside A, Diana A, Oikonomidou O. Lobular Breast Cancer: A Review. Front Oncol. 2021 Jan 15;10 PMID: 33520704
4 Berx G, Cleton-Jansen AM, Nollet F, de Leeuw WJ, van de Vijver M, Cornelisse C, van Roy F. E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. EMBO J. 1995 Dec 15;14(24):6107-15. doi: 10.1002/j.1460-2075.1995.tb00301.x. PMID: 8557030; PMCID: PMC394735.
5 Derksen PW, Liu X, Saridin F, van der Gulden H, Zevenhoven J, Evers B, van Beijnum JR, Griffioen AW, Vink J, Krimpenfort P, Peterse JL, Cardiff RD, Berns A, Jonkers J. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006 Nov;10(5):437-49. doi: 10.1016/j.ccr.2006.09.013. PMID: 17097565.
6 Newcomer LM, Newcomb PA, Trentham-Dietz A, Storer BE, Yasui Y, Daling JR, Potter JD. Detection method and breast carcinoma histology. Cancer. 2002 Aug 1;95(3):470-7. PMID: 12209738.
7Desmedt C, Zoppoli G, Gundem G, Pruneri G, Larsimont D, Fornili M, Fumagalli D, Brown D, Rothé F, Vincent D, Kheddoumi N, Rouas G, Majjaj S, Brohée S, Van Loo P, Maisonneuve P, Salgado R, Van Brussel T, Lambrechts D, Bose R, Metzger O, Galant C, Bertucci F, Piccart-Gebhart M, Viale G, Biganzoli E, Campbell PJ, Sotiriou C. Genomic Characterization of Primary Invasive Lobular Breast Cancer. J Clin Oncol. 2016 Jun 1;34(16):1872-81. doi: 10.1200/JCO.2015.64.0334. Epub 2016 Feb 29. PMID: 26926684.
8Michaut M, Chin SF, Majewski I, Severson TM, Bismeijer T, de Koning L, Peeters JK, Schouten PC, Rueda OM, Bosma AJ, Tarrant F, Fan Y, He B, Xue Z, Mittempergher L, Kluin RJ, Heijmans J, Snel M, Pereira B, Schlicker A, Provenzano E, Ali HR, Gaber A, O’Hurley G, Lehn S, Muris JJ, Wesseling J, Kay E, Sammut SJ, Bardwell HA, Barbet AS, Bard F, Lecerf C, O’Connor DP, Vis DJ, Benes CH, McDermott U, Garnett MJ, Simon IM, Jirström K, Dubois T, Linn SC, Gallagher WM, Wessels LF, Caldas C, Bernards R. Integration of genomic, transcriptomic and proteomic data identifies two biologically distinct subtypes of invasive lobular breast cancer. Sci Rep. 2016 Jan 5;6:18517. doi: 10.1038/srep18517. PMID: 26729235; PMCID: PMC4700448.
9 Li CI, Anderson BO, Daling JR, Moe RE. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003 Mar 19;289(11):1421-4. doi: 10.1001/jama.289.11.1421. PMID: 12636465
10 Reference no longer current. See reference 11.
11Adapted 2018 ACS Surveillance Research, SEER
12Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021 Jan;71(1):7-33. Epub 2021 Jan 12. Erratum in: CA Cancer J Clin. 2021 Jul;71(4):359. PMID: 33433946.
13Christgen M, Cserni G, Floris G, Marchio C, Djerroudi L, Kreipe H, Derksen PWB, Vincent-Salomon A. Lobular Breast Cancer: Histomorphology and Different Concepts of a Special Spectrum of Tumors. Cancers (Basel). 2021 Jul 22;13(15):3695. PMID: 34359596
14 Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6(3):R149-56. doi: 10.1186/bcr767. Epub 2004 Feb 17. PMID: 15084238; PMCID: PMC400666.
15Findlay-Shirras LJ, Lima I, Smith G, Clemons M, Arnaout A. Population Trends in Lobular Carcinoma of the Breast: The Ontario Experience. Ann Surg Oncol. 2020 Nov;27(12):4711-4719. PMID: 32725525.
16Pestalozzi BC, Zahrieh D, Mallon E, Gusterson BA, Price KN, Gelber RD, Holmberg SB, Lindtner J, Snyder R, Thürlimann B, Murray E, Viale G, Castiglione-Gertsch M, Coates AS, Goldhirsch A; International Breast Cancer Study Group. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol. 2008 Jun 20;26(18):3006-14. doi: 10.1200/JCO.2007.14.9336. Epub 2008 May 5. PMID: 18458044.
17 https://lobularbreastcancer.org/wp-content/uploads/2022/10/Symptoms_of_ILC_2022.pdf
18Corso G, Intra M, Trentin C, Veronesi P, Galimberti V. CDH1 germline mutations and hereditary lobular breast cancer. Fam Cancer. 2016 Apr;15(2):215-9. PMID: 26759166.
19 https://www.nccn.org/patients/guidelines/content/PDF/breast-invasive-patient.pdf
20 Manahan ER, Kuerer HM, Sebastian M, Hughes KS, Boughey JC, Euhus DM, Boolbol SK, Taylor WA. Consensus Guidelines on Genetic Testing for Hereditary Breast Cancer from the American Society of Breast Surgeons. Ann Surg Oncol. 2019 Oct;26(10):3025-3031. Epub 2019 Jul 24. PMID: 31342359.
21 Daly MB, Pal T, Berry MP, Buys SS, et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021 Jan 6;19(1):77-102. PMID: 33406487.
22 Corso G, Figueiredo J, La Vecchia C, Veronesi P, Pravettoni G, Macis D, Karam R, Lo Gullo R, Provenzano E, Toesca A, Mazzocco K, Carneiro F, Seruca R, Melo S, Schmitt F, Roviello F, De Scalzi AM, Intra M, Feroce I, De Camilli E, Villardita MG, Trentin C, De Lorenzi F, Bonanni B, Galimberti V. Hereditary lobular breast cancer with an emphasis on E-cadherin genetic defect. J Med Genet. 2018 Jul;55(7):431-441. Epub 2018 Jun 21. PMID: 29929997.
23 Petridis C, Arora I, Shah V, Moss CL, Mera A, Clifford A, Gillett C, Pinder SE, Tomlinson I, Roylance R, Simpson MA, Sawyer EJ. Frequency of Pathogenic Germline Variants in CDH1, BRCA2, CHEK2, PALB2, BRCA1, and TP53 in Sporadic Lobular Breast Cancer. Cancer Epidemiol Biomarkers Prev. 2019 Jul;28(7):1162-1168. PMID: 31263054.
24 King TA, Pilewskie M, Muhsen S, Patil S, Mautner SK, Park A, Oskar S, Guerini-Rocco E, Boafo C, Gooch JC, De Brot M, Reis-Filho JS, Morrogh M, Andrade VP, Sakr RA, Morrow M. Lobular Carcinoma in Situ: A 29-Year Longitudinal Experience Evaluating Clinicopathologic Features and Breast Cancer Risk. J Clin Oncol. 2015 Nov 20;33(33):3945-52. doi: 10.1200/JCO.2015.61.4743. Epub 2015 Sep 14. PMID: 26371145; PMCID: PMC4934644.
25 Schnitt SJ, Brogi E, Chen YY, King TA, Lakhani SR. American Registry of Pathology Expert Opinions: The Spectrum of Lobular Carcinoma in Situ: Diagnostic Features and Clinical Implications. Ann Diagn Pathol. 2020 Apr;45:151481. PMID: 32120324
26 Hillary Stires, Rebecca B. Riggins. The role of androgen receptor in invasive lobular breast carcinoma [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2017; 2017 Apr 1-5; Washington, DC. Philadelphia (PA): AACR; Cancer Res 2017;77(13 Suppl):Abstract nr 3605. doi:10.1158/1538-7445.AM2017-3605
27 Bergeron, A., MacGrogan, G., Bertaut, A. et al. Triple-negative breast lobular carcinoma: a luminal androgen receptor carcinoma with specific ESRRA mutations. Mod Pathol 34, 1282–1296 (2021). https://doi.org/10.1038/s41379-021-00742-9
28 Miglietta F, et al. Evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer. 2021; 7:137
29 https://densebreast-info.org/wp-content/uploads/2022/12/Patient-Fact-Sheet-English1222.pdf
30 Li CI, Weiss NS, Stanford JL, Daling JR. Hormone replacement therapy in relation to risk of lobular and ductal breast carcinoma in middle-aged women. Cancer. 2000 Jun 1;88(11):2570-7. PMID: 10861435.
31 Berg WA, Gutierrez L, NessAiver MS, Carter WB, Bhargavan M, Lewis RS, Ioffe OB. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004 Dec;233(3):830-49. PMID: 15486214.
32 Wecsler J, Jeong YJ, Raghavendra AS, Mack WJ, Tripathy D, Yamashita MW, Sheth PA, Hovanessian Larsen L, Russell CA, MacDonald H, Sener SF, Lang JE. Factors associated with MRI detection of occult lesions in newly diagnosed breast cancers. J Surg Oncol. 2020 Mar;121(4):589-598. PMID: 31984517
33 Parvaiz MA, Yang P, Razia E, Mascarenhas M, Deacon C, Matey P, Isgar B, Sircar T. Breast MRI in Invasive Lobular Carcinoma: A Useful Investigation in Surgical Planning? Breast J. 2016 Mar-Apr;22(2):143-50. PMID: 26841281.
34 Ha SM, Chae EY, Cha JH, Kim HH, Shin HJ, Choi WJ. Breast MR Imaging before Surgery: Outcomes in Patients with Invasive Lobular Carcinoma by Using Propensity Score Matching. Radiology. 2018 Jun;287(3):771-777. PMID: 29388904.
35 Sung JS, Lebron L, Keating D, D’Alessio D, Comstock CE, Lee CH, Pike MC, Ayhan M, Moskowitz CS, Morris EA, Jochelson MS. Performance of Dual-Energy Contrast-enhanced Digital Mammography for Screening Women at Increased Risk of Breast Cancer. Radiology. 2019 Oct;293(1):81-88. doi: 10.1148/radiol.2019182660. Epub 2019 Aug 27. PMID: 31453765; PMCID: PMC6776233.
36 Sorin V, Yagil Y, Yosepovich A, Shalmon A, Gotlieb M, Neiman OH, Sklair-Levy M. Contrast-Enhanced Spectral Mammography in Women With Intermediate Breast Cancer Risk and Dense Breasts. AJR Am J Roentgenol. 2018 Nov;211(5):W267-W274. doi: 10.2214/AJR.17.19355. Epub 2018 Sep 21. PMID: 30240292.
37 Covington MF, Parent EE, Dibble EH, Rauch GM, Fowler AM. Advances and Future Directions in Molecular Breast Imaging. J Nucl Med. 2022 Jan;63(1):17-21. doi: 10.2967/jnumed.121.261988. PMID: 34887334
38 Dibble EH, Hunt KN, Ehman EC, O’Connor MK. Molecular Breast Imaging in Clinical Practice. AJR Am J Roentgenol. 2020 Aug;215(2):277-284. Epub 2020 Jun 17. PMID: 32551908.
39 Houssami N, Abraham LA, Miglioretti DL, et al. Accuracy and Outcomes of Screening Mammography in Women With a Personal History of Early-Stage Breast Cancer. JAMA. 2011;305(8):790–799. doi:10.1001/jama.2011.188
40 Mukhtar RA, Wong J, Piper M, Zhu Z, Fahrner-Scott K, Mamounas M, Sbitany H, Alvarado M, Foster R, Ewing C, Esserman L. Breast Conservation and Negative Margins in Invasive Lobular Carcinoma: The Impact of Oncoplastic Surgery and Shave Margins in 358 Patients. Ann Surg Oncol. 2018 Oct;25(11):3165-3170. Epub 2018 Jul 27. PMID: 30054826.
41 Luveta J, Parks RM, Heery DM, Cheung KL, Johnston SJ. Invasive Lobular Breast Cancer as a Distinct Disease: Implications for Therapeutic Strategy. Oncol Ther. 2020 Jun;8(1):1-11. Epub 2019 Dec 24. PMID: 32700069
42 Fodor J, Major T, Tóth J, Sulyok Z, Polgár C. Comparison of mastectomy with breast-conserving surgery in invasive lobular carcinoma: 15-Year results. Rep Pract Oncol Radiother. 2011 Jul 27;16(6):227-31. PMID: 24376985
43 Abel, MK, Brabha, CE, Guo R, Fahrner-Scott K, Wong J, Alvarado M, Ewing C, Esserman LJ, Mukhtar RA, Breast conservation therapy versus mastectomy in the surgical management of invasive lobular carcinoma measuring 4 cm or greater. The American Journal of Surgery. 2021 Jan. 221: 32-32. DOI:https://doi.org/10.1016/j.amjsurg.2020.05.038
44 Vicini FA, Cecchini RS, White JR, et al Primary results of NSABP B-39/RTOG 0413 (NRG Oncology): A randomized phase III study of conventional whole breast irradiation (WBI) versus partial breast irradiation (PBI) for women with stage 0, I, or II breast cancer [abstract]. In: Proceedings of the 2018 San Antonio Breast Cancer Symposium; 2018 Dec 4-8; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2019;79(4 Suppl):Abstract nr GS4-04.
45 Crown A, Rocha FG, Grumley JW. Intraoperative radiation therapy in early-stage breast cancer: Presence of lobular features is not associated with increased rate of requiring additional therapy. Am J Surg. 2020 Jul;220(1):161-164. PMID: 31839176.
46 Metzger Filho O, Giobbie-Hurder A, Mallon E, Gusterson B, et al A. Relative Effectiveness of Letrozole Compared With Tamoxifen for Patients With Lobular Carcinoma in the BIG 1-98 Trial. J Clin Oncol. 2015 Sep 1;33(25):2772-9. PMID: 26215945
47 van Hellemond IEG, Geurts SME, Tjan-Heijnen VCG. Current Status of Extended Adjuvant Endocrine Therapy in Early Stage Breast Cancer. Curr Treat Options Oncol. 2018 Apr 27;19(5):26. PMID: 29704066
48 Noordhoek I, Treuner K, Putter H, Zhang Y, Wong J, Meershoek-Klein Kranenbarg E, Duijm-de Carpentier M, van de Velde CJH, Schnabel CA, Liefers GJ. Breast Cancer Index Predicts Extended Endocrine Benefit to Individualize Selection of Patients with HR+ Early-stage Breast Cancer for 10 Years of Endocrine Therapy. Clin Cancer Res. 2021 Jan 1;27(1):311-319. PMID: 33109739.
49 Felts JL, Zhu J, Han B, Smith SJ, Truica CI. An Analysis of Oncotype DX Recurrence Scores and Clinicopathologic Characteristics in Invasive Lobular Breast Cancer. Breast J. 2017 Nov;23(6):677-686. PMID: 28097781.
50 Thornton MJ, Williamson HV, Westbrook KE, Greenup RA, Plichta JK, Rosenberger LH, Gupta AM, Hyslop T, Hwang ES, Fayanju OM. Neoadjuvant Endocrine Therapy Versus Neoadjuvant Chemotherapy in Node-Positive Invasive Lobular Carcinoma. Ann Surg Oncol. 2019 Oct;26(10):3166-3177. PMID: 31342392
51 Barroso-Sousa R, Metzger-Filho O. Differences between invasive lobular and invasive ductal carcinoma of the breast: results and therapeutic implications. Ther Adv Med Oncol. 2016 Jul;8(4):261-6. PMID: 27482285
52 Riba LA, Russell T, Alapati A, Davis RB, James TA. Characterizing Response to Neoadjuvant Chemotherapy in Invasive Lobular Breast Carcinoma. J Surg Res. 2019 Jan;233:436-443. doi: 10.1016/j.jss.2018.08.011. Epub 2018 Sep 21. PMID: 30502283. https://pubmed.ncbi.nlm.nih.gov/30502283/
53 Johnston SRD, Harbeck N, Hegg R; monarchE Committee Members and Investigators. Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J Clin Oncol. 2020 Dec 1;38(34):3987-3998. PMID: 32954927
54 Cortesi L, Rugo HS, Jackisch C. An Overview of PARP Inhibitors for the Treatment of Breast Cancer. Target Oncol. 2021 May;16(3):255-282. PMID: 33710534
55 Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, Gelber RD, de Azambuja E, Fielding A, Balmaña J, Domchek SM, Gelmon KA, Hollingsworth SJ, Korde LA, Linderholm B, Bandos H, Senkus E, Suga JM, Shao Z, Pippas AW, Nowecki Z, Huzarski T, Ganz PA, Lucas PC, Baker N, Loibl S, McConnell R, Piccart M, Schmutzler R, Steger GG, Costantino JP, Arahmani A, Wolmark N, McFadden E, Karantza V, Lakhani SR, Yothers G, Campbell C, Geyer CE Jr; OlympiA Clinical Trial Steering Committee and Investigators. Adjuvant Olaparib for Patients with BRCA1– or BRCA2-Mutated Breast Cancer. N Engl J Med. 2021 Jun 24;384(25):2394-2405. doi: 10.1056/NEJMoa2105215. Epub 2021 Jun 3. PMID: 34081848; PMCID: PMC9126186.
56 Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, Lee KS, Niikura N, Park YH, Xu B, Wang X, Gil-Gil M, Li W, Pierga JY, Im SA, Moore HCF, Rugo HS, Yerushalmi R, Zagouri F, Gombos A, Kim SB, Liu Q, Luo T, Saura C, Schmid P, Sun T, Gambhire D, Yung L, Wang Y, Singh J, Vitazka P, Meinhardt G, Harbeck N, Cameron DA; DESTINY-Breast04 Trial Investigators. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med. 2022 Jul 7;387(1):9-20. doi: 10.1056/NEJMoa2203690. Epub 2022 Jun 5. PMID: 35665782.
57 Ambrosone CB, Zirpoli GR, Hutson AD, McCann WE, McCann SE, Barlow WE, Kelly KM, Cannioto R, Sucheston-Campbell LE, Hershman DL, Unger JM, Moore HCF, Stewart JA, Isaacs C, Hobday TJ, Salim M, Hortobagyi GN, Gralow JR, Budd GT, Albain KS. Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients With Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221). J Clin Oncol. 2020 Mar 10;38(8):804-814. PMID: 31855498
58 Spei ME, Samoli E, Bravi F, La Vecchia C, Bamia C, Benetou V. Physical activity in breast cancer survivors: A systematic review and meta-analysis on overall and breast cancer survival. Breast. 2019 Apr;44:144-152. doi: 10.1016/j.breast.2019.02.001. Epub 2019 Feb 12. PMID: 30780085.
59 https://www.cancer.gov/about-cancer/treatment/cam
60 Han E, Johnson N, DelaMelena T, Glissmeyer M, Steinbock K. Alternative therapy used as primary treatment for breast cancer negatively impacts outcomes. Ann Surg Oncol. 2011 Apr;18(4):912-6. doi: 10.1245/s10434-010-1487-0. Epub 2011 Jan 12. PMID: 21225354.
61 Chang EY, Glissmeyer M, Tonnes S, Hudson T, Johnson N. Outcomes of breast cancer in patients who use alternative therapies as primary treatment. Am J Surg. 2006 Oct;192(4):471-3. doi: 10.1016/j.amjsurg.2006.05.013. PMID: 16978951.
62 Joseph, K., Vrouwe, S., Kamruzzaman, A. et al. Outcome analysis of breast cancer patients who declined evidence-based treatment. World J Surg Onc 10, 118 (2012). https://doi.org/10.1186/1477-7819-10-118
63 Saquib J, Parker BA, Natarajan L, Madlensky L, Saquib N, Patterson RE, Newman VA, Pierce JP. Prognosis following the use of complementary and alternative medicine in women diagnosed with breast cancer. Complement Ther Med. 2012 Oct;20(5):283-90. doi: 10.1016/j.ctim.2012.04.002. Epub 2012 Apr 27. PMID: 22863642; PMCID: PMC3413169.
64 Johnson SB, Park HS, Gross CP, Yu JB. Complementary Medicine, Refusal of Conventional Cancer Therapy, and Survival Among Patients With Curable Cancers. JAMA Oncol. 2018 Oct 1;4(10):1375-1381. doi: 10.1001/jamaoncol.2018.2487. PMID: 30027204; PMCID: PMC6233773.
65 Mouabbi, J.A., Hassan, A., Lim, B. et al. Invasive lobular carcinoma: an understudied emergent subtype of breast cancer. Breast Cancer Res Treat (2022). https://link.springer.com/epdf/10.1007/s10549-022-06572-w?sharing_token=jfKacO89Z0HFMApWZGBI4Pe4RwlQNchNByi7wbcMAY5RZaLmt158Qrv-z7KGSYmJYCjQDvt8mUmM0cLZDOw-glCNrZvd6xQqZoJHYeMu8CnzKspbdOmzy_JfiUtVyIUkNGMMDeOBbLHAVkUZgIZE1g4UJ–7kAXu6rQihrDTTJA%3D
66 Franzoi MA, Hortobagyi GN. Leptomeningeal carcinomatosis in patients with breast cancer. Crit Rev Oncol Hematol. 2019 Mar;135:85-94. doi: 10.1016/j.critrevonc.2019.01.020. Epub 2019 Feb 1. PMID: 30819451.
67 Blohmer M, Zhu L, Atkinson JM, Beriwal S, Rodríguez-López JL, Rosenzweig M, Brufsky AM, Tseng G, Lucas PC, Lee AV, Oesterreich S, Jankowitz RC. Patient treatment and outcome after breast cancer orbital and periorbital metastases: a comprehensive case series including analysis of lobular versus ductal tumor histology. Breast Cancer Res. 2020 Jun 26;22(1):70. doi: 10.1186/s13058-020-01309-3. PMID: 32586354; PMCID: PMC7318761.
68 He H, Gonzalez A, Robinson E, Yang WT. Distant metastatic disease manifestations in infiltrating lobular carcinoma of the breast. AJR Am J Roentgenol. 2014 May;202(5):1140-8. doi: 10.2214/AJR.13.11156. PMID: 24758672.
69 Mathew A, Rajagopal PS, Villgran V, Sandhu GS, Jankowitz RC, Jacob M, Rosenzweig M, Oesterreich S, Brufsky A. Distinct Pattern of Metastases in Patients with Invasive Lobular Carcinoma of the Breast. Geburtshilfe Frauenheilkd. 2017 Jun;77(6):660-666. Epub 2017 Jun 28. PMID: 28757653
70 Hogan MP, Goldman DA, Dashevsky B, Riedl CC, Gönen M, Osborne JR, Jochelson M, Hudis C, Morrow M, Ulaner GA. Comparison of 18F-FDG PET/CT for Systemic Staging of Newly Diagnosed Invasive Lobular Carcinoma Versus Invasive Ductal Carcinoma. J Nucl Med. 2015 Nov;56(11):1674-80. Epub 2015 Aug 20. PMID: 26294295.
71 Pesapane F, Downey K, Rotili A, Cassano E, Koh DM. Imaging diagnosis of metastatic breast cancer. Insights Imaging. 2020 Jun 16;11(1):79. doi: 10.1186/s13244-020-00885-4. PMID: 32548731; PMCID: PMC7297923.
72 https://lobularbreastcancer.org/ilc-clinical-trials/
73 Ulaner GA, Jhaveri K, Chandarlapaty S, Hatzoglou V, Riedl CC, Lewis JS, Mauguen A. Head-to-Head Evaluation of 18F-FES and 18F-FDG PET/CT in Metastatic Invasive Lobular Breast Cancer. J Nucl Med. 2021 Mar;62(3):326-331. doi: 10.2967/jnumed.120.247882. Epub 2020 Jul 17. PMID: 32680923; PMCID: PMC8049349.
74 Bhaludin BN, Tunariu N, Koh DM, Messiou C, Okines AF, McGrath SE, Ring AE, Parton MM, Sharma B, Gagliardi T, Allen SD, Pope R, Johnston SRD, Downey K. A review on the added value of whole-body MRI in metastatic lobular breast cancer. Eur Radiol. 2022 Sep;32(9):6514-6525. doi: 10.1007/s00330-022-08714-6. Epub 2022 Apr 6. PMID: 35384456.
75 Gao JJ, Cheng J, Bloomquist E, et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2020 Feb;21(2):250-260. Epub 2019 Dec 16. PMID: 31859246.
76 Pérez-Garcia J, Cortés J, Metzger Filho O. Efficacy of Single-Agent Chemotherapy for Patients with Advanced Invasive Lobular Carcinoma: A Pooled Analysis from Three Clinical Trials. Oncologist. 2019 Aug;24(8):1041-1047. Epub 2018 Dec 21. PMID: 30578311
77 https://www.cancer.net/cancer-types/breast-cancer-metastatic/types-treatment
78 Cardoso MJ, Mokbel K. Locoregional therapy in de novo metastatic breast cancer. The unanswered question. Breast. 2021 Aug;58:170-172. Epub 2021 May 7. PMID: 34158166
79 Khan et al. Khan SA, Zhao F, Goldstein LJ, Cella D, Basik M, Golshan M, Julian TB, Pockaj BA, Lee CA, Razaq W, Sparano JA, Babiera GV, Dy IA, Jain S, Silverman P, Fisher CS, Tevaarwerk AJ, Wagner LI, Sledge GW. Early Local Therapy for the Primary Site in De Novo Stage IV Breast Cancer: Results of a Randomized Clinical Trial (EA2108). J Clin Oncol. 2022 Mar 20;40(9):978-987. PMID: 34995128
80 Soran A, Dogan L, Isik A, Ozbas S, Trabulus DC, Demirci U, Karanlik H, Soyder A, Dag A, Bilici A, Dogan M, Koksal H, Sendur MAN, Gulcelik MA, Maralcan G, Cabioglu N, Yeniay L, Utkan Z, Simsek T, Karadurmus N, Daglar G, Yildiz B, Uras C, Tukenmez M, Yildirim A, Kutun S, Ozaslan C, Karaman N, Akcay MN, Toktas O, Sezgin E. The Effect of Primary Surgery in Patients with De Novo Stage IV Breast Cancer with Bone Metastasis Only (Protocol BOMET MF 14-01): A Multi-Center, Prospective Registry Study. Ann Surg Oncol. 2021 Sep;28(9):5048-5057.. PMID: 33532878.
81 Bilani N, Yaghi M, Main O, Naik M, Jabbal I, Rivera C, Elson L, Liang H, Saravia D, Nahleh Z. Metastasectomy versus radiation of secondary sites in stage IV breast cancer: Analysis from a national cancer registry. Breast. 2021 Dec;60:185-191. PMID: 34673385
82 Kurozumi S, Alsaleem M, Monteiro CJ, et al Targetable ERBB2 mutation status is an independent marker of adverse prognosis in estrogen receptor positive, ERBB2 non-amplified primary lobular breast carcinoma: a retrospective in silico analysis of public datasets. Breast Cancer Res. 2020 Aug 11;22(1):85 PMID: 32782013
83 Ma C, Luo J, Freedman R, et al. A phase II trial of neratinib (NER) or NER plus fulvestrant (FUL) (N+F) in HER2 mutant, non-amplified (HERmut) metastatic breast cancer (MBC): Part II of MutHER. Cancer Res. 2021;81(suppl 13):CT026. doi:10.1158/1538- 7445.
84 Komal Jhaveri, Cristina Saura, Angel Guerrero-Zotano, et al Latest findings from the breast cancer cohort in SUMMIT – a phase 2 ‘basket’ trial of neratinib + trastuzumab + fulvestrant for HER2-mutant, hormone receptor-positive, metastatic breast cancer [abstract]. In: Proceedings of the 2020 San Antonio Breast Cancer Virtual Symposium; 2020 Dec 8-11; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2021;81(4 Suppl):Abstract nr PD1-05.https://clinicaltrials.gov/ct2/show/NCT01953926
85 Desmedt, C., Pingitore, J., Rothé, F. et al. ESR1 mutations in metastatic lobular breast cancer patients. npj Breast Cancer 5, 9 (2019). https://doi.org/10.1038/s41523-019-0104-z
86 Seth A. Wander, Ofir Cohen, Xueqian Gong, Gabriela N. Johnson, Jorge Buendia-Buendia, Maxwell Lloyd, Dewey Kim, Flora Luo, Pingping Mao, Karla Helvie, Kailey Kowalski, Utthara Nayar, Stephen Parsons, Ricardo Martinez, Lacey Litchfield, Xiang Ye, Chun Ping Yu, Valerie Jansen, Levi A. Garraway, Eric P. Winer, Sara M. Tolaney, Nancy U. Lin, Sean Buchanan, Nikhil Wagle. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) in patients with hormone receptor-positive (HR+)/HER2- metastatic breast cancer (MBC) [abstract]. In: Proceedings of the 2019 San Antonio Breast Cancer Symposium; 2019 Dec 10-14; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2020;80(4 Suppl):Abstract nr PD2-09.
87 Mukhtar, R.A., Chien, A.J. Invasive Lobular Carcinoma of the Breast: Ongoing Trials, Challenges, and Future Directions. Curr Breast Cancer Rep (2021). https://rdcu.be/cKTyP
88 Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, Hayes W, Hodi FS, Hoekstra OS, Huang EP, Lin N, Liu Y, Therasse P, Wolchok JD, Seymour L. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer. 2016 Jul;62:132-7. doi: 10.1016/j.ejca.2016.03.081. Epub 2016 May 14. PMID: 27189322; PMCID: PMC5737828.
89 Abel MK, Melisko ME, Rugo HS, Chien AJ, Diaz I, Levine JK, Griffin A, McGuire J, Esserman LJ, Borno HT, Mukhtar RA. Decreased enrollment in breast cancer trials by histologic subtype: does invasive lobular carcinoma resist RECIST? NPJ Breast Cancer. 2021 Oct 25;7(1):139. doi: 10.1038/s41523-021-00348-z. PMID: 34697300; PMCID: PMC8547221.
Please note: This FAQ is for informational and educational purposes only. Information found on these pages or links should never replace professional medical advice.