LBCA Scientific Advisor Dr. Gary Ulaner Describes Promising Methods in Clinical Trials for Better ILC Detection
November 19, 2020
I am a diagnostic radiologist and nuclear medicine physician with expertise in translating novel Positron Emission Tomography (PET) agents to assist patients with cancer, particularly breast cancer. I am very excited by the possibilities for PET imaging to help stage, localize disease, select optimal therapies, and measure treatment response in patients with breast cancer.

There are many types of imaging that can be utilized for patients with breast cancer. Mammography, breast ultrasound, and breast magnetic resonance (MR) are all utilized to evaluate the disease within the breast and local lymph nodes. This imaging is incredibly important for making treatment decisions. I am more focused on imaging disease that has spread beyond the breast and local nodes. The presence of disease beyond the breast and local nodes, often called “distant metastases” is important as well. For patients at initial diagnosis, imaging detection of distant metastases greatly impacts their staging, choice of therapies, and prognosis. For patients already with known distant metastases, imaging helps determine response to therapy and can even help select the therapies that are used.
So, what is the best way to image distant metastases? Unfortunately, this is not a question with an easy, or even a well agreed upon, answer. The method currently suggested by the National Comprehensive Cancer Network (NCCN) are two tests called computed tomography (CT) and bone scan. The CT can detect disease in many sites, including nodes, lung, and liver, however, it is not very sensitive at detecting disease in the bone. (A “sensitive” test can find disease early, while a less sensitive test may miss disease until the disease is larger or more advanced.) The bone scan is used to augment the CT scan, as it can help detect bone disease earlier than a CT.
I work on PET imaging methods that are often more sensitive for detecting disease than CT/bone scan. PET is an imaging method where a molecule that goes to a biologic process (such as cancer) is “labeled” with a small amount of radioactivity. This amount of radioactivity is not enough to treat the tumor or cause noticeable side effects in the patient, but is enough for the molecule to be localized by an imaging machine called a PET scanner. An overview of how a cancer cell expresses a target that can be detected by a PET imaging agent is shown in Figure 1.
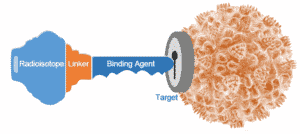
I work with a number of PET imaging agents. 18F-fluorodeoxyglucose (FDG) is the most commonly used PET imaging agent. It is a sugar molecule labeled with the radioisotope Fluorine-18. The “target” in this case is sugar metabolism. Since most tumors use lots of sugar, FDG is very effective at imaging many tumors. FDG is often more sensitive than CT/bone scan for imaging metastases in patients with breast cancer.
One limitation of FDG s that not all tumors use the same amount of sugar. Tumors that do not use much sugar are harder to detect with FDG. Invasive lobular breast cancer (ILC) is one type of breast cancer that often does not use much sugar. This is one of the differences between ILC and the more common ductal breast cancer (IDC) that tends to use more sugar. To overcome limitations such as this, researchers are working on the development of other PET radiotracers. Two of the PET radiotracers I work with have shown potential for imaging ILC. The first is Fluciclovine (18F-FACBC), which “targets” amino acid metabolism. We and others have shown that Fluciclovine may more sensitively detect ILC metastases than other imaging methods (Ulaner et al, Journal of Nuclear Medicine, 2016). There is an upcoming prospective clinical trial to be led by Dr. David Schuster, Director of Nuclear Medicine and Molecular Imaging at Emory University School of Medicine in Atlanta, Georgia, to further test Fluciclovine in ILC. I feel fortunate that I will be part of that trial.
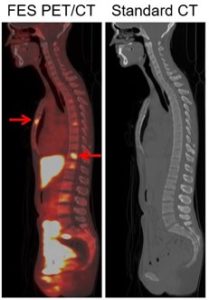
The second is FES (18F-fluoroestradiol), which targets the estrogen receptor (ER). As ILC is nearly always ER-positive, targeting the ER is an appealing method for imaging ILC. While we are evaluating FES in both ILC and IDC, as ILC is harder to find on other imaging modalities, we expect there to be a greater relative advantage of FES in ILC. We have published studies of FES in breast cancer, both IDC and ILC, demonstrating its potential (Wang et al, Clinical Cancer Research, 2017; Ulaner et al, Journal of Nuclear Medicine, 2021). An example from this research is shown in Figure 2.
We are currently preparing to open a new prospective clinical trial* of FES for patients with breast cancer at the Hoag Family Cancer Institute in Newport Beach and Irvine, California.
I hope our research adds to the growing efforts to help patients with ILC. To all the patients, advocates, health care providers and researchers in the field, I send best wishes for a brighter future.
Interested in hearing more from Dr. Ulaner? Click here to watch his video.
* This trial is now open.

